Chapter 14 Reading Question 10 Organic Chemistry
Grignard Reagents Are Awesome: Their Formation, Reactions, And Reaction Mechanisms
In a blatant plug for the Reagent Guide and the Reagents App for iPhone, each Friday I profile a different reagent that is commonly encountered in Org 1/ Org 2.

Today'south reagent is one that most students have experience in making at some point or another. Grignard reagents are formed past the reaction of magnesium metal with alkyl or alkenyl halides. They're extremely good nucleophiles, reacting with electrophiles such every bit carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. They're also very strong bases and will react with acidic hydrogens (such every bit alcohols, h2o, and carboxylic acids).
Similar to or the aforementioned every bit: very similar to organolithium reagents.
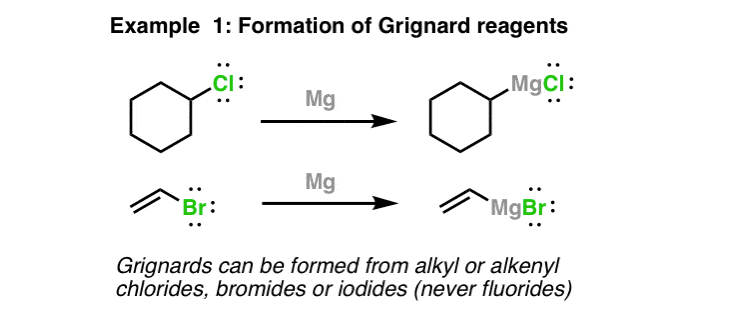
Formation Of Grignard Reagents
Grignard reagents are made through the addition of magnesium metallic to alkyl or alkenyl halides. The halide tin be Cl, Br, or I (non F). It'south slightly easier to make Grignards from the iodides and bromides, however. Note what'south happening hither – the magnesium is "inserting" itself between the carbon and the halide. This halide the "X" referred to when nosotros refer to Grignard reagents as "RMgX".
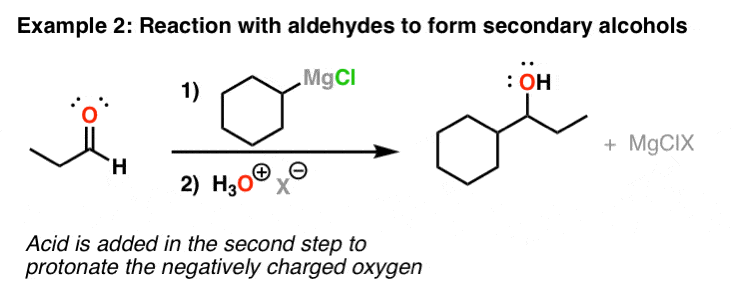
Reaction Of Grignard Reagents With Aldehydes To Give Secondary Alcohols
I of the almost common uses of Grignard reagents is in their reaction with aldehydes and ketones to course alcohols. In the get-go footstep, the Grignard forms the carbon-carbon bond. This results in an alkoxide (the conjugate base of an booze). To form the alcohol, it's necessary to add acid at the end of the reaction (in what'due south called the "workup" step). This is shown here as "H3O+" (the "X" is but the counter-ion, a spectator here)
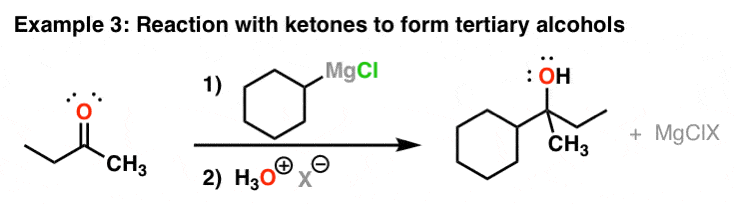
Reaction Of Grignard Reagents With Ketones To Give Tertiary Alcohols
The reaction behaves similarly with ketones. Again, there's nothing special most the Cl hither – information technology all depends on how you made the Grignard in the first identify.
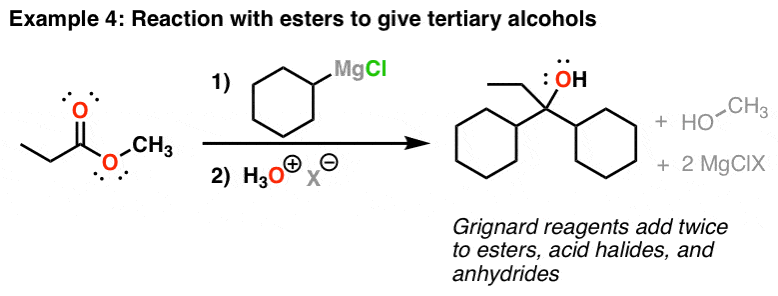
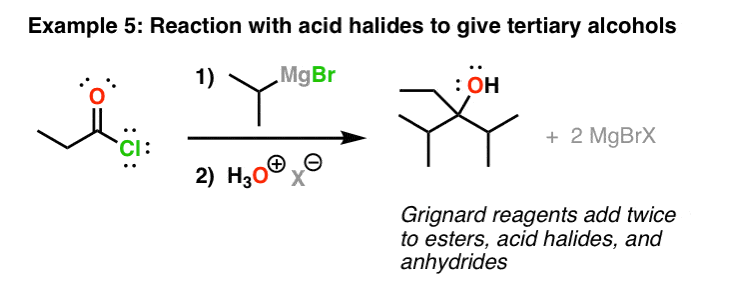
Grignards Add Twice To Esters And Acid Halides
Grignard reagents will also add to esters. What makes these reactions a little more complicated is that they add twice. The cyberspace result (later on addition of acid) is a tertiary alcohol. This is also the case for acid halides (acyl halides) and anhydrides. One notable exception is carboxylic acids (more on that below).
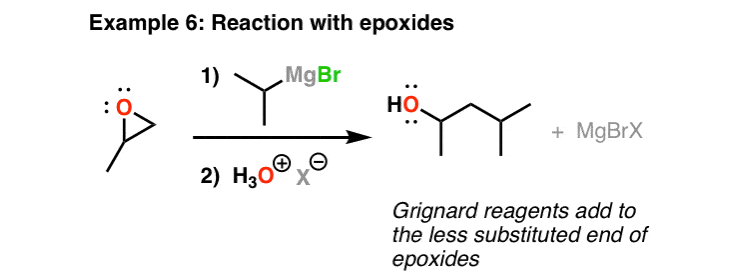
Addition Of Grignard Reagents To Epoxides
Another important reaction of Grignard reagents is that they will add together to epoxides to grade carbon-carbon bonds. One matter to keep in mind here is that the trend is for them to add to the less substituted end of the epoxide – that is, the less sterically hindered cease. You can remember of this reaction as being essentially similar to an SN2 reaction. After addition of acid, an alcohol is obtained.
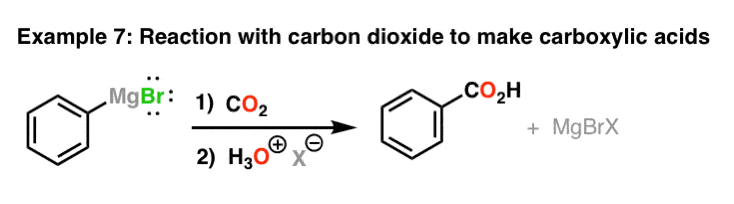
Reaction Of Grignard Reagents With Carbon Dioxide To Give Carboxylic Acids
Grignard reagents also add to carbon dioxide (CO2) to class carboxylates, in a reaction like to their reactions with ketones and aldehydes. The carboxylates are converted to carboxylic acids after add-on of acid (such as our trusty H3O(+) ).
Grignard Reagents Are Strong Bases – Protonation (And Deuteration)
Finally, since Grignard reagents are essentially the cohabit bases of alkanes, they're also extremely strong bases. This means that sometimes acid-base reactions tin can compete with their nucleophilic improver reactions. One common situation where this crops upwards is when Grignard reagents are added to carboxylic acids. It'south easy to forget that carboxylic acids… are acids. This ways that instead of adding to the carbonyl, they react with the proton instead and form the carboxylate common salt.
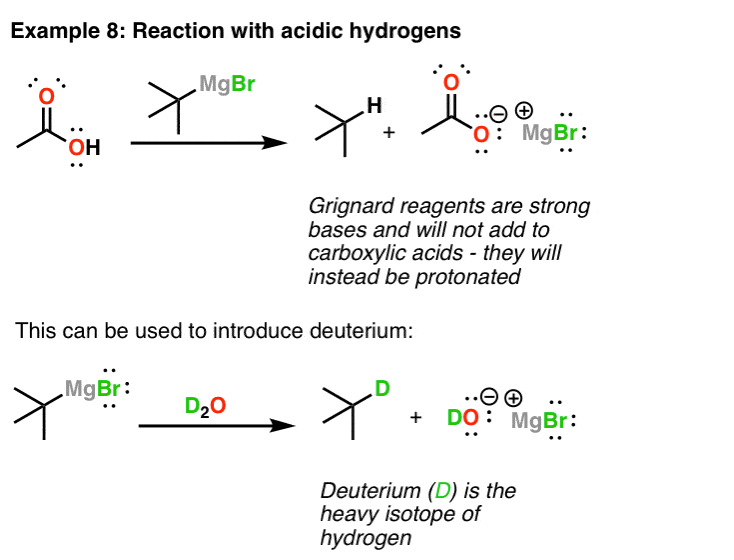
This can also be used to convert alkyl halides to alkanes. Showtime yous treat it with magnesium, so you treat the Grignard with a potent acid. This gives y'all the alkane. Yous can also apply this to introduce deuterium (D) into molecules! The first step is to make the Grignard reagent. The second is to treat that Grignard with a deuterated acrid such as D2O. This gives you the deuterated alkane!
Mechanism: Addition Of Grignard Reagents To Aldehydes And Ketones
And so how does information technology work? The cardinal to the Grignard reagent is actually very unproblematic. When you lot think about the relative electronegativities of carbon (2.v) and magnesium (1.1), the bond between carbon and magnesium is polarized toward carbon. That means that carbon is more electron rich than magnesium and is really nucleophilic! Here's a closer await.
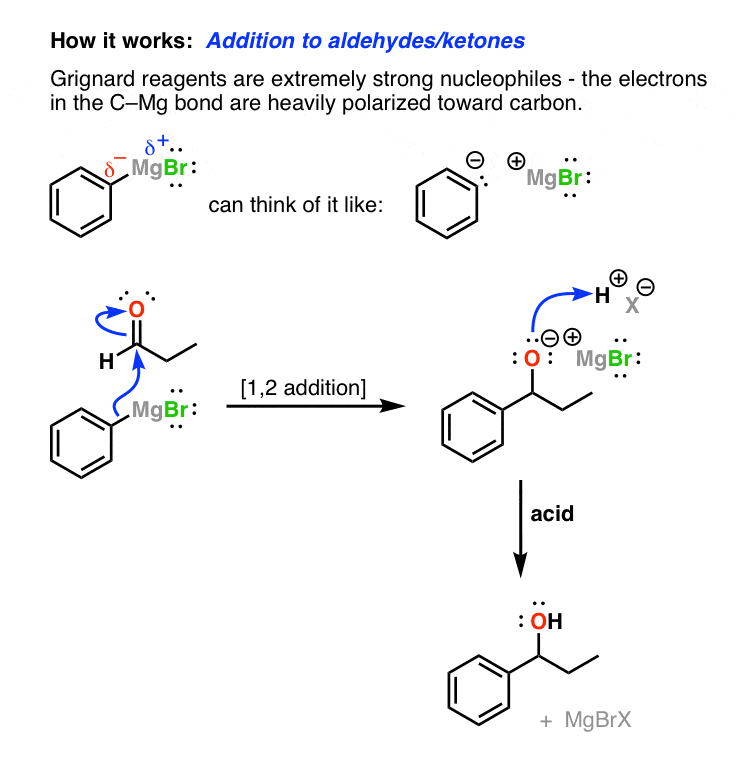
In the reaction of Grignards with aldehydes, the carbon attacks the carbonyl carbon and performs a ane,two-addition to give an alkoxide. In the second step, acid is added to requite you lot the alcohol.
There are so many other elements to the Grignard but a express amount of infinite. So I'll leave it in that location. If you desire more details you'll have to check out the Reagent Guide!
P.Due south. Yous can read well-nigh the chemistry of Grignard reagents and more than 80 other reagents in undergraduate organic chemical science in the "Organic Chemical science Reagent Guide", available here equally a downloadable PDF. The Reagents App is also bachelor for iPhone, click on the icon beneath!
![]()
(Advanced) References and Further Reading
- Grignard, 5. C. Acad. Sci. 1900, 130, 1322-1324
The original paper past Victor Grignard describing a new method for alcohol synthesis from hydrocarbons. - Victor Grignard and Paul Sabatier: Two Showcase Laureates of the Nobel Prize for Chemistry
Henri B. Kagan
Chem. Int. Ed. 2012, 51, 2-9
DOI: 10.1002/anie.201201849
For those interested in the history of science, this is a historical perspective on the lives of Victor Grignard and Paul Sabatier, and gives insight into their lives, how they made their seminal discoveries, and the impact of their piece of work, among other things. - Mechanical activation of magnesium turnings for the preparation of reactive Grignard reagents
Karen V. Baker, John M. Brown, Nigel Hughes, A. Jerome Skarnulis, and Ann Sexton
The Periodical of Organic Chemistry 1991 56 (ii), 698-703
DOI: ten.1021/jo00002a039
Sometimes the formation of a Grignard reagent using Mg metal tin can exist challenging, and various methods for activating the metal surface accept been developed, including mechanical activation by dry-stirring Mg turnings under an inert temper for several hours.
The post-obit three papers are mechanistic studies on the germination of Grignard reagents: - The Mechanism of Formation of Grignard Reagents: Trapping of Gratis Alkyl Radical Intermediates by Reaction with Tetramethylpiperidine-Northward-oxyl
Karen Due south. Root, Craig 50. Colina, Lynette Grand. Lawrence, and George M. Whitesides
Journal of the American Chemical Social club 1989 111 (xiv), 5405-5412
DOI: 10.1021/ja00196a053 - Mechanism of Grignard Reagent Formation. The Surface Nature of the Reaction
M. Walborsky and Janusz Rachon
Periodical of the American Chemical Lodge 1989 111 (5), 1896-1897
DOI: 10.1021/ja00187a063 - Machinery of Grignard Reagent Formation. Comparisons of D-Model Calculations with Experimental Production Yields
John F. Garst and Brian Fifty. Swift
Periodical of the American Chemic Gild 1989 111 (ane), 241-250
DOI: 10.1021/ja00183a037 - 4-METHOXY-iv′-NITROBIPHENYL
K. Stille, Antonio M. Echavarren, Robert M. Williams, and James A. Hendrix
Org. Synth. 1993, 71, 97
DOI: 10.15227/orgsyn.071.0097
The second pace in this procedure includes the synthesis of p-anisylmagnesium bromide, which can be a tricky Grignard reagent to set up and requires special activation of Mg with methyl iodide.
Source: https://www.masterorganicchemistry.com/2011/10/14/reagent-friday-grignard-reagents/
0 Response to "Chapter 14 Reading Question 10 Organic Chemistry"
Post a Comment